Land-based Industrialized Recirculating Aquaculture System (RAS) Process and Parameter Design (Part 3): Water Quality Parameters
Recirculating Water Quality Parameters
Water quality parameters and design standards form the basis for recirculating water treatment system design and operational management. Below are reference diagrams and parameters commonly used by the Engineering Team:
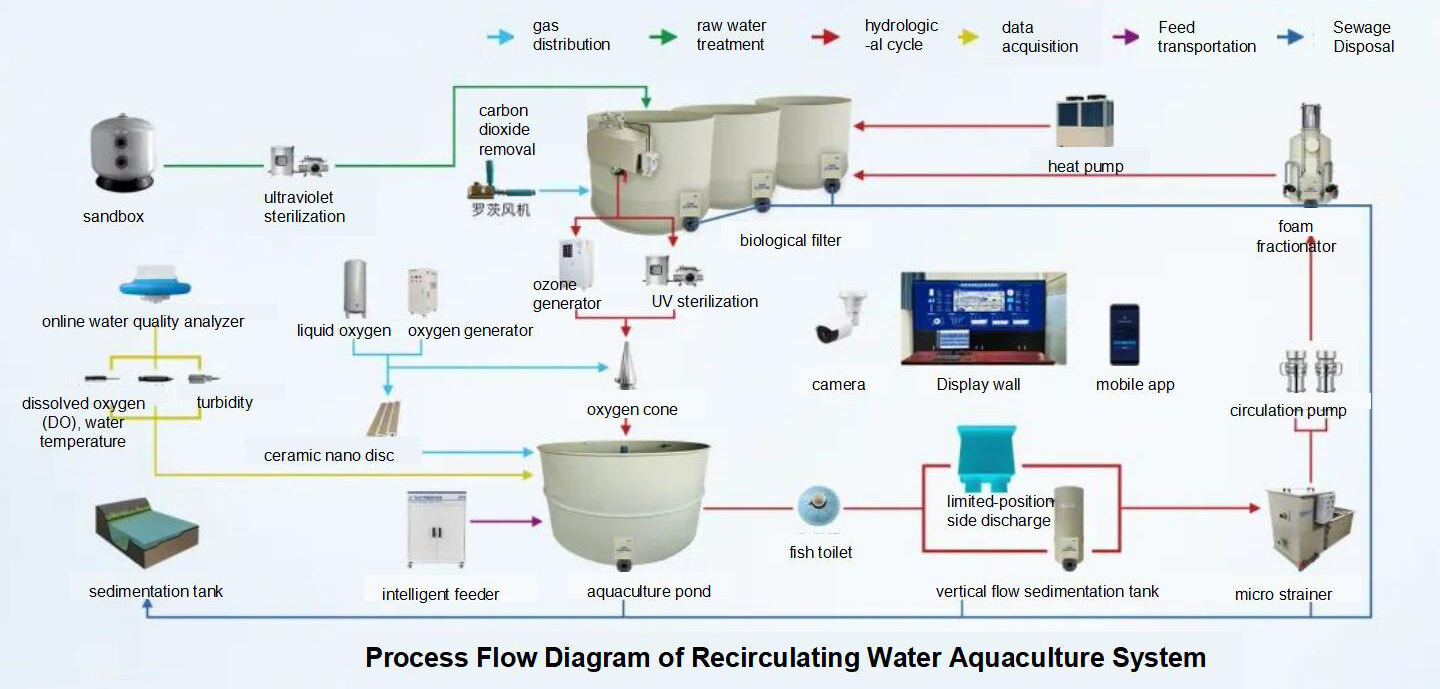
|
Water Quality Parameters |
|
|
Total Suspended Solids (TSS) |
≤10mg/L |
|
Total Ammonia Nitrogen (TAN) |
≤1mg/L |
|
Nitrite (NO₂⁻ - N) |
≤0.5mg/L |
|
Nitrate (NO₃⁻ - N) |
≤300mg/L |
|
Dissolved Oxygen (DO) |
8-10mg/L |
|
pH |
7-8.5 |
|
Oxidation-Reduction Potential (ORP) |
≤400mV |
|
Water Temperature |
23-30℃ |
1. Solid Particle Removal System Design
Total Suspended Solids (TSS) is commonly used as a parameter to measure solid particulate matter in Recirculating Aquaculture System (RAS). It mainly refers to the total amount of solid particles with a particle size greater than 1 micron in a unit of water. In the circulating water system, TSS includes fish feces, residual bait, biological flocs (dead and live bacteria), etc. The size of these suspended particles varies greatly from micrometer to centimeter level. Suspended particulate matter can directly affect the health and growth of fish (especially cold water fish), and also increase the burden on biofilters. Therefore, it is necessary to maintain the concentration of suspended particles in the circulating water within a reasonable range.
In some EU countries' Recirculating Aquaculture System (RAS) systems, the control of suspended particulate matter is relatively strict. For example, for water bodies used for Recirculating Aquaculture System (RAS), the concentration of suspended particulate matter (measured by total suspended solids TSS) is usually expected to be controlled below 15mg/L to maintain good water quality and ecological environment.
The United States also has relevant water quality regulations in the fields of aquaculture and water treatment. In the Recirculating Aquaculture System (RAS) system, the corresponding suspended particulate matter content (converted by turbidity and other related indicators) also has certain limitations. The ideal range for suspended particulate matter concentration is around 8-12 mg/L, which is used to ensure the survival and reproduction of aquatic organisms.
In the actual operation of the factory based Recirculating Aquaculture System (RAS) system in China, it is generally required to control the suspended particulate matter concentration (suspended solids SS) below 10mg/L. For some precious species that require high water quality, such as salmon, it is even required to control it below 5mg/L.

2. Dissolved Contaminant Removal Parameters
Water solubility includes soluble inorganic substances and soluble organic substances. Among them, water-soluble harmful substances are mainly ammonia nitrogen (NH3-N) and nitrite nitrogen (NO2-- N). Ammonia nitrogen can enter the bloodstream through the gills and skin of fish, disrupting their normal tricarboxylic acid cycle, altering their osmotic pressure, and reducing their ability to absorb oxygen from water, thus affecting their normal growth and survival.
The commonly used fixed membrane nitrification biofilter in Recirculating Aquaculture System (RAS) systems is the ammonia nitrogen conversion bacterial community that grows on the surface of a certain biological packing material, and ammonia nitrogen is transferred to the fixed biofilm through diffusion and converted. The main purpose of the biological filter process design is to ensure that the filter has sufficient nitrifying bacteria to remove ammonia nitrogen excreted by fish, maintain the ammonia nitrogen concentration in the aquaculture system within the predetermined range, and ensure the safety and effective growth of fish.
2.1 Ammonia Nitrogen (NH₃-N) Control
Ammonia nitrogen is one of the main pollutants dissolved in water in Recirculating Aquaculture System (RAS) systems. It mainly comes from the excrement and residual feed of farmed organisms. High concentrations of ammonia nitrogen can be toxic to farmed organisms, affecting their growth, immunity, and reproductive ability. In biofilters, the removal of ammonia nitrogen mainly relies on the nitrification of microorganisms such as nitrifying bacteria, which convert ammonia nitrogen into nitrite and nitrate.
When designing a biofilter, sufficient surface area and volume of the filter material should be considered to provide enough space for nitrifying bacteria to grow and reproduce. At the same time, it is necessary to control the ammonia nitrogen load in the influent and avoid excessive ammonia nitrogen concentration from impacting the biological filter. For example, the concentration of ammonia nitrogen in the influent can be reduced by using an automatic feeding machine and adopting a feeding strategy of small amounts and multiple meals. Determine the allowable ammonia nitrogen concentration for the biofilter based on the ammonia nitrogen tolerance and breeding density of the cultured organisms. Generally speaking, for most freshwater aquaculture fish, the total ammonia nitrogen concentration should be controlled below 1mg/L, and non-ionic ammonia should not exceed 0.025mg/L.
2.2 Nitrite (NO₂⁻-N) Control
Nitrite is also a water quality parameter that needs to be closely monitored in the Recirculating Aquaculture System (RAS) system. It is an intermediate product in the process of ammonia nitrogen nitrification and is also toxic to aquaculture organisms. Nitrite can affect the transport of oxygen in the blood of farmed organisms, leading to symptoms of hypoxia such as shortness of breath, floating heads, and even death.
In the design, it is necessary to ensure that the biofilter can effectively further convert nitrite into nitrate. This requires maintaining the activity of denitrifying bacteria in the biofilter and providing them with suitable environmental conditions, such as appropriate dissolved oxygen. Generally, it is required to control the concentration of nitrite below 0.5mg/L.
2.3 Seawater Aquaculture Considerations
The salinity of seawater is relatively high, containing various ions such as sodium ions (Na ⁺), chloride ions (Cl ⁻), magnesium ions (Mg ² ⁺), calcium ions (Ca ² ⁺), etc. Marine aquaculture organisms have evolved complex ion regulation systems during their long-term adaptation to high salt environments. When nitrite enters marine organisms, these organisms can partially alleviate the physiological effects of nitrite by utilizing their own ion regulation system. In Recirculating Aquaculture System (RAS), chloride ions (Cl -) can reduce the toxicity of nitrite (NO2-) to aquaculture organisms through competitive inhibition. Specifically, both chloride ions and nitrite need to enter the fish body through the chloride cells on the gill plates. The presence of chloride ions increases the difficulty of nitrite entering the fish body, thereby reducing its toxicity. In general, when the concentration of chloride ions in water is six times that of nitrite, it can effectively inhibit the toxicity of nitrite to aquaculture organisms. Compared to freshwater aquaculture, seawater aquaculture has less toxic hazards from nitrite, which is related to the higher concentration of chloride ions in seawater. Therefore, in the Recirculating Aquaculture System (RAS) system, by regulating salinity reasonably, the toxicity of nitrite can be effectively reduced, and the health and safety of aquaculture organisms can be protected.
3. Dissolved Oxygen (DO)
In a Recirculating Aquaculture System (RAS) system, dissolved oxygen (DO) is a key water quality parameter. Fish and other aquatic organisms absorb dissolved oxygen from water through gill respiration to maintain their metabolic activity. The dissolved oxygen concentration required for the normal growth of most warm water fish is generally around 5-8mg/L. When the dissolved oxygen concentration is below the critical level, the respiration of aquatic organisms will be inhibited, their growth rate will slow down, their immunity will decrease, and they are prone to infection with diseases. For example, when the dissolved oxygen is below 2mg/L, many fish will experience floating head phenomenon, and prolonged exposure to low dissolved oxygen can lead to fish death.
In Recirculating Aquaculture System (RAS), it is recommended to maintain dissolved oxygen between 8-10 mg/L. Higher dissolved oxygen is beneficial for increasing feeding levels and reducing feed to feed ratios.
4. pH Control
In a Recirculating Aquaculture System (RAS) system, the suitable pH range for fish is usually between 7.0-8.5. For example, most freshwater fish grow well in environments with pH 7.2-7.8. This is because within this pH range, the physiological functions of fish, such as respiration and osmotic pressure regulation, can be carried out relatively normally. Gas exchange occurs through the gills, and the appropriate acidity or alkalinity in water facilitates the normal exchange process of oxygen and carbon dioxide.
For shrimp farming, such as South American white shrimp, the suitable pH range is roughly 7.8-8.6. This is due to the physiological structure and activity characteristics of crustaceans, which make them more adaptable to slightly higher pH environments. Suitable pH is beneficial for the molting growth of shrimp.
However, during the process of Recirculating Aquaculture System (RAS), the pH value will continuously decrease as the aquaculture progresses, and it is necessary to adjust the pH value of the water. Automatic pH adjustment equipment can be used. Automatically adjust the pH value of the water body based on pH sensor data.
Recommended Products
Hot News
-
Is it true that raising fish in high-density canvas fish ponds is more efficient than ordinary ponds?
2024-12-16
-
Advantages of galvanized canvas fish pond
2024-10-14
-
High-density fish farming technology, fish pond cost, canvas fish pond, canvas pond, high-density fish farming
2024-10-12
-
Why choose flowing water high-density aquaculture
2023-11-20















































